Innovative working groups prove useful in facilitating finalization of AMR texts on time
The final session of the Ad hoc Codex Intergovernmental Task Force on Antimicrobial Resistance (TFAMR8) will take place virtually from 4 to 9 October 2021, with the adoption of the report scheduled for 13 October. Mandated by the Codex Alimentarius Commission in 2016, the Task Force is to:
- revise the Code of Practice to Minimize and Contain Antimicrobial Resistance (CXC 61-2005) (COP) – and
- develop a new document: Guidance on Integrated Monitoring and Surveillance of Antimicrobial Resistance (GLIS) to address the management of foodborne AMR along the food chain.
Both texts are aimed to address the management of foodborne AMR along the food chain and to incorporate the One Health approach, taking into account the work of relevant international organizations, such as FAO, WHO and OIE.
When the COVID-19 pandemic put all Codex physical meetings and international travel on hold, the Codex Secretariat AMR team, led by Gracia Brisco, immediately began to explore how to bring countries together to keep the Task Force momentum going. Webinars in January and April 2021 provided Codex Members and Observers with an update on the work taking place in the electronic working groups (EWG) and explained where effort was still needed to bring the texts closer to completion. “We felt it critical to give as much space for constructive discussion as possible, so that the different points of view could be heard as we come close to the end of the time allotted to the Task Force,” said Brisco.
Working groups on the COP and GLIS meeting virtually
Codex technical committees often make use of working groups held, when Codex is functioning normally, just before the formal plenary week-long session. These physical meetings, usually chaired by countries who have been leading the work, allow for specific issues and obstacles to be ironed out before the full committee aims to reach final agreement.
For TFAMR these working groups had to take place virtually in June 2021 and with time running out countries have had to swiftly adapt to the online format.
The co-chairs’ in-depth analyses and preparations in advance of the meetings have to be given huge credit.
Manisha Mehrotra, Canada, said: “The working groups were excellent and the meeting format has given an opportunity to advance the two documents at the next Task Force meeting. I would say in particular for the GLIS working group, the co-chairs’ in-depth analyses and preparations in advance of the meetings have to be given huge credit! In addition, a high level of participation by Members and delegations being well-prepared for the discussions have been instrumental in making progress on the texts.”
The working groups gave Members an opportunity to express their views on some issues that had only been dealt with in electronic working groups. “This also allows other Members that have not been participating in the EWGs to understand and many times support some of the different views,” said Suzana Breslau and Ligia Schreiner from Brazil.
Kjersti Nilsen Barkbu, Norway, said: “Discussing issues where differences need to be resolved are more challenging when meeting virtually. It is harder to reach consensus when we do not have the informal coffee break or breakfast talk.”
Although the working groups have made some progress, much remains to be done at the TFAMR Session in October, 2021 says Mabel Aworh, Nigeria. “The virtual working environment has created many challenges including limitations to the ability to communicate freely and share ideas, technical issues, and time zone issues for some delegations,” she said.
Inspiring participation.
Harriet Ofori, Ghana, described the participation of Member Countries as ‘inspiring’, considering the constraints imposed by the pandemic and problems of internet connectivity encountered in some countries. “It is our hope that near normal times would return to enable physical meetings to improve Member participation as countries work together to support the progress of these global documents,” she said.
“The working groups have been very helpful - indeed have done much of the work of the Task Force. The challenge for TFAMR is to not allow some countries who didn't get the exact wording they wanted - to go back to text which was broadly agreed in the working groups,” said Carel du Marchie Sarvaas from HealthforAnimals, a Codex Observer.
Steve Roach, who works at Food Animal Concerns Trust in the United States and is part of the Consumers International observer delegation, also feels some of the work is going well. “I think we were able to come to decent agreement on the code of practice and we should be able to complete the remaining work. On the GLIS text, the chairs have done a remarkable [job] trying to move the document forward despite the tight deadlines and big differences among Members,” he said.
What differences remain to be resolved?
Code of practice
There are issues where there is polarity in views with delegations leaning towards either deleting, keeping or revising specific text in the draft documents. “In the code of practice, this revolves around the inclusion of ‘therapeutic/therapy’ in the document and the formulation of Principle 13, “said Manisha Mehrotra.
To complete the code of practice in Norway’s view it is crucial to resolving the differences regarding the definition of ‘therapeutic use’. “There was not enough discussion on the key elements ... [and] due to this we were not able to discuss similar wording in other places in the code, which we would have anticipated. Therefore, the work did not progress as much as we would have hoped for,” said Nilsen Barkbu.
A great advance for the prevention and control of AMR.
“For this document, Brazil is of the opinion that it should be adopted, and countries that do not agree on any specific issue because of their national or regional legislation should place a reservation. This document is a great advance for the prevention and control of AMR and all the work already done should not be wasted,” said Breslau and Schreiner.
Mabel Aworh, Nigeria, believes there are numerous challenges to reaching consensus as in the need to include the definition of therapeutic and principles including therapeutic in the code of practice. “Consensus could be achieved if Members are willing to be flexible and take into account that some countries use different words to describe similar practices to others,” she said.
“On content, success will occur when those who always oppose the same texts, start proposing alternative compromise texts which they think might work for others,” said du Marchie Sarvaas.
Nairobi, Kenya - Members of the AMR Surveillance Pilot Study among chicken layering farmers within Kiambu County analyze samples in a laboratory.
GLIS
There was no opportunity to discuss the GLIS text at the last TFAMR plenary held in Pyeongchang, Republic of Korea in December 2019 and the time that the working group has spent to review and revise the document, with in depth review and discussions of key sections in the text, will hopefully pave the way for a smoother discussion in plenary.
Canada believes consensus on the new Codex guidance on surveillance of AMR revolves primarily around the inclusion of guidance on surveillance of antimicrobial use (AMU). “I think these issues can be resolved with a common willingness to try and find text in the middle, for example, for AMU, perhaps compromise could consist of text that provides more flexibility to countries in the implementation of the guidelines. There have been significant discussions at the recently concluded meetings and I am optimistic that countries would maintain that momentum to find happy middle ground,” said Mehrotra.
According to Brazil, many Members understand that the section of the document regarding AMU (Section 9) should only have high level recommendations and refer to organizations that are already dealing with this topic, related to their mandate. “While we do not advance in these parts of the document it is difficult to have an overview of the whole document, as parts of other sections will be influenced by the decisions we make for this part. There is still a huge challenge ahead of us if we ambitiously want to finalize it at TFAMR8,” said Breslau and Schreiner.
For Aworh, the GLIS attracts more consensus challenges especially on scientific issues and methods. “It is particularly important that the GLIS is clear and practicable for developing countries to implement as a majority rely on Codex texts. The GLIS should use language that is flexible as opposed to being too prescriptive because resources are limited hence the need to prioritize what can be achieved,” she said.
On whether or not AMU should be included in the text, Aworh said: “It is important to note that AMU is not the only driver for AMR so it should not carry the same weight in the GLIS as AMR. Also given that AMU is not in the initial terms of reference and is already being monitored by the World Organisation for Animal Health (OIE), a solution could be either removing references to AMU from the GLIS, or significantly limiting the text dedicated to it. This could enable consensus. On this issue, the co-chairs were able to propose a compromise on some paragraphs and tried to reduce the text significantly with reference made to the OIE.”
For Roach, the challenges really revolve around how much detail to include in the document and some countries' concerns about impacts on trade. “There also seems to be confusion among Members on the appropriate roles of the three international standard setting bodies with some Members wanting to ignore the Codex responsibility to address the threat of antimicrobial resistance in the food supply and let the other two bodies deal with it. The problem with this approach is that Codex is the only organization with primary responsibility for the safety of food. For myself, this is disappointing because the focus of Codex in developing standards should be consumer protection and that too often gets lost when countries focus on protecting access to markets,” he said.
Spirit of compromise
So will both texts make it to the final stage of the Codex Step process and be put forward for adoption by the Commission in November 2021?
For some, the consequences of not reaching agreement on the code of practice would undermine the efforts by many Members who have already worked hard and made a lot of compromise.
TFAMR would be setting a pretty poor example, believes Mehrotra, particularly on a topic that is a high priority at the international level. “We have made significant progress over the past few years and I am hopeful that there will be constructive ideas at plenary to resolve the very few remaining issues,” she said.
It is important that Codex take part in the common fight to combat AMR.
It would be considered a defeat for Codex not being able to bring Members together to globally fight the development of AMR in Norway’s opinion. “In our view, it is important that Codex take part in the common fight to combat AMR. Therefore, we hope all the efforts made by the TFAMR will enable us to reach an agreement on the text.”
Harriet Ofori, Ghana, sees the need to work more in the spirit of compromise considering the differing conditions in countries in terms of the knowledge, data and resources necessary to address the problem of antimicrobial resistance. “We believe that differences can be resolved easily should a thought be given to the above recommendations,” she said.
A clear opportunity to deliver on its mandate.
Gracia Brisco has proved that a creative, inclusive approach to international negotiation on pressing topics, harnessing the experience of the Codex Secretariat with the global expertise in technical committees has provided Members and the Task Force with a clear opportunity to deliver on its mandate. “Countries and observers have now had multiple opportunities to achieve consensus on the texts even though working virtually and with all the challenges remote discussions bring. I expect countries to continue in this positive and constructive vein when we come to final discussions at TFAMR8,” she said.
Read more
TFAMR8 dedicated web pages including access to the latest drafts of the two texts
Q & A with Rosa Peran, The Netherlands, Chairperson of the GLIS EWG available in English, French and Spanish.
Q & A with Donald Prater, USA, Chairperson of the COP EWG available in English, French and Spanish.
Photo credit
©FAO/Luis Tato / FAO
At the heart of the Codex mandate are the core values of collaboration, inclusiveness, consensus building and transparency. Governmental and non-governmental, public and private organizations alike play a vital role in ensuring Codex texts are of the highest quality and based on sound science.
Codex would have little authority in the field of international standard setting if it did not welcome and acknowledge the valuable contributions made by observers. Expert technical bodies, industry and consumer associations
contribute to the standard-setting process in a spirit of openness, collaboration and transparency.
Intergovernmental organizations (IGOs) and international non-governmental organizations (NGOs) can apply for observer status in Codex in order to attend and put forward their views at every stage of the standard-setting process.
 Current Codex Alimentarius Commission
Current Codex Alimentarius Commission
Innovative working groups prove useful in facilitating finalization of AMR texts on time
The final session of the Ad hoc Codex Intergovernmental Task Force on Antimicrobial Resistance (TFAMR8) will take place virtually from 4 to 9 October 2021, with the adoption of the report scheduled for 13 October. Mandated by the Codex Alimentarius Commission in 2016, the Task Force is to:
- revise the Code of Practice to Minimize and Contain Antimicrobial Resistance (CXC 61-2005) (COP) – and
- develop a new document: Guidance on Integrated Monitoring and Surveillance of Antimicrobial Resistance (GLIS) to address the management of foodborne AMR along the food chain.
Both texts are aimed to address the management of foodborne AMR along the food chain and to incorporate the One Health approach, taking into account the work of relevant international organizations, such as FAO, WHO and OIE.
When the COVID-19 pandemic put all Codex physical meetings and international travel on hold, the Codex Secretariat AMR team, led by Gracia Brisco, immediately began to explore how to bring countries together to keep the Task Force momentum going. Webinars in January and April 2021 provided Codex Members and Observers with an update on the work taking place in the electronic working groups (EWG) and explained where effort was still needed to bring the texts closer to completion. “We felt it critical to give as much space for constructive discussion as possible, so that the different points of view could be heard as we come close to the end of the time allotted to the Task Force,” said Brisco.
Working groups on the COP and GLIS meeting virtually
Codex technical committees often make use of working groups held, when Codex is functioning normally, just before the formal plenary week-long session. These physical meetings, usually chaired by countries who have been leading the work, allow for specific issues and obstacles to be ironed out before the full committee aims to reach final agreement.
For TFAMR these working groups had to take place virtually in June 2021 and with time running out countries have had to swiftly adapt to the online format.
The co-chairs’ in-depth analyses and preparations in advance of the meetings have to be given huge credit.
Manisha Mehrotra, Canada, said: “The working groups were excellent and the meeting format has given an opportunity to advance the two documents at the next Task Force meeting. I would say in particular for the GLIS working group, the co-chairs’ in-depth analyses and preparations in advance of the meetings have to be given huge credit! In addition, a high level of participation by Members and delegations being well-prepared for the discussions have been instrumental in making progress on the texts.”
The working groups gave Members an opportunity to express their views on some issues that had only been dealt with in electronic working groups. “This also allows other Members that have not been participating in the EWGs to understand and many times support some of the different views,” said Suzana Breslau and Ligia Schreiner from Brazil.
Kjersti Nilsen Barkbu, Norway, said: “Discussing issues where differences need to be resolved are more challenging when meeting virtually. It is harder to reach consensus when we do not have the informal coffee break or breakfast talk.”
Although the working groups have made some progress, much remains to be done at the TFAMR Session in October, 2021 says Mabel Aworh, Nigeria. “The virtual working environment has created many challenges including limitations to the ability to communicate freely and share ideas, technical issues, and time zone issues for some delegations,” she said.
Inspiring participation.
Harriet Ofori, Ghana, described the participation of Member Countries as ‘inspiring’, considering the constraints imposed by the pandemic and problems of internet connectivity encountered in some countries. “It is our hope that near normal times would return to enable physical meetings to improve Member participation as countries work together to support the progress of these global documents,” she said.
“The working groups have been very helpful - indeed have done much of the work of the Task Force. The challenge for TFAMR is to not allow some countries who didn't get the exact wording they wanted - to go back to text which was broadly agreed in the working groups,” said Carel du Marchie Sarvaas from HealthforAnimals, a Codex Observer.
Steve Roach, who works at Food Animal Concerns Trust in the United States and is part of the Consumers International observer delegation, also feels some of the work is going well. “I think we were able to come to decent agreement on the code of practice and we should be able to complete the remaining work. On the GLIS text, the chairs have done a remarkable [job] trying to move the document forward despite the tight deadlines and big differences among Members,” he said.
What differences remain to be resolved?
Code of practice
There are issues where there is polarity in views with delegations leaning towards either deleting, keeping or revising specific text in the draft documents. “In the code of practice, this revolves around the inclusion of ‘therapeutic/therapy’ in the document and the formulation of Principle 13, “said Manisha Mehrotra.
To complete the code of practice in Norway’s view it is crucial to resolving the differences regarding the definition of ‘therapeutic use’. “There was not enough discussion on the key elements ... [and] due to this we were not able to discuss similar wording in other places in the code, which we would have anticipated. Therefore, the work did not progress as much as we would have hoped for,” said Nilsen Barkbu.
A great advance for the prevention and control of AMR.
“For this document, Brazil is of the opinion that it should be adopted, and countries that do not agree on any specific issue because of their national or regional legislation should place a reservation. This document is a great advance for the prevention and control of AMR and all the work already done should not be wasted,” said Breslau and Schreiner.
Mabel Aworh, Nigeria, believes there are numerous challenges to reaching consensus as in the need to include the definition of therapeutic and principles including therapeutic in the code of practice. “Consensus could be achieved if Members are willing to be flexible and take into account that some countries use different words to describe similar practices to others,” she said.
“On content, success will occur when those who always oppose the same texts, start proposing alternative compromise texts which they think might work for others,” said du Marchie Sarvaas.
Nairobi, Kenya - Members of the AMR Surveillance Pilot Study among chicken layering farmers within Kiambu County analyze samples in a laboratory.
GLIS
There was no opportunity to discuss the GLIS text at the last TFAMR plenary held in Pyeongchang, Republic of Korea in December 2019 and the time that the working group has spent to review and revise the document, with in depth review and discussions of key sections in the text, will hopefully pave the way for a smoother discussion in plenary.
Canada believes consensus on the new Codex guidance on surveillance of AMR revolves primarily around the inclusion of guidance on surveillance of antimicrobial use (AMU). “I think these issues can be resolved with a common willingness to try and find text in the middle, for example, for AMU, perhaps compromise could consist of text that provides more flexibility to countries in the implementation of the guidelines. There have been significant discussions at the recently concluded meetings and I am optimistic that countries would maintain that momentum to find happy middle ground,” said Mehrotra.
According to Brazil, many Members understand that the section of the document regarding AMU (Section 9) should only have high level recommendations and refer to organizations that are already dealing with this topic, related to their mandate. “While we do not advance in these parts of the document it is difficult to have an overview of the whole document, as parts of other sections will be influenced by the decisions we make for this part. There is still a huge challenge ahead of us if we ambitiously want to finalize it at TFAMR8,” said Breslau and Schreiner.
For Aworh, the GLIS attracts more consensus challenges especially on scientific issues and methods. “It is particularly important that the GLIS is clear and practicable for developing countries to implement as a majority rely on Codex texts. The GLIS should use language that is flexible as opposed to being too prescriptive because resources are limited hence the need to prioritize what can be achieved,” she said.
On whether or not AMU should be included in the text, Aworh said: “It is important to note that AMU is not the only driver for AMR so it should not carry the same weight in the GLIS as AMR. Also given that AMU is not in the initial terms of reference and is already being monitored by the World Organisation for Animal Health (OIE), a solution could be either removing references to AMU from the GLIS, or significantly limiting the text dedicated to it. This could enable consensus. On this issue, the co-chairs were able to propose a compromise on some paragraphs and tried to reduce the text significantly with reference made to the OIE.”
For Roach, the challenges really revolve around how much detail to include in the document and some countries' concerns about impacts on trade. “There also seems to be confusion among Members on the appropriate roles of the three international standard setting bodies with some Members wanting to ignore the Codex responsibility to address the threat of antimicrobial resistance in the food supply and let the other two bodies deal with it. The problem with this approach is that Codex is the only organization with primary responsibility for the safety of food. For myself, this is disappointing because the focus of Codex in developing standards should be consumer protection and that too often gets lost when countries focus on protecting access to markets,” he said.
Spirit of compromise
So will both texts make it to the final stage of the Codex Step process and be put forward for adoption by the Commission in November 2021?
For some, the consequences of not reaching agreement on the code of practice would undermine the efforts by many Members who have already worked hard and made a lot of compromise.
TFAMR would be setting a pretty poor example, believes Mehrotra, particularly on a topic that is a high priority at the international level. “We have made significant progress over the past few years and I am hopeful that there will be constructive ideas at plenary to resolve the very few remaining issues,” she said.
It is important that Codex take part in the common fight to combat AMR.
It would be considered a defeat for Codex not being able to bring Members together to globally fight the development of AMR in Norway’s opinion. “In our view, it is important that Codex take part in the common fight to combat AMR. Therefore, we hope all the efforts made by the TFAMR will enable us to reach an agreement on the text.”
Harriet Ofori, Ghana, sees the need to work more in the spirit of compromise considering the differing conditions in countries in terms of the knowledge, data and resources necessary to address the problem of antimicrobial resistance. “We believe that differences can be resolved easily should a thought be given to the above recommendations,” she said.
A clear opportunity to deliver on its mandate.
Gracia Brisco has proved that a creative, inclusive approach to international negotiation on pressing topics, harnessing the experience of the Codex Secretariat with the global expertise in technical committees has provided Members and the Task Force with a clear opportunity to deliver on its mandate. “Countries and observers have now had multiple opportunities to achieve consensus on the texts even though working virtually and with all the challenges remote discussions bring. I expect countries to continue in this positive and constructive vein when we come to final discussions at TFAMR8,” she said.
Read more
TFAMR8 dedicated web pages including access to the latest drafts of the two texts
Q & A with Rosa Peran, The Netherlands, Chairperson of the GLIS EWG available in English, French and Spanish.
Q & A with Donald Prater, USA, Chairperson of the COP EWG available in English, French and Spanish.
Photo credit
©FAO/Luis Tato / FAO
 Codex and Observer
Codex and Observer
around the world since ancient times.
We might not always know where it comes from,
but we expect it to be available, safe and of good quality.







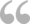
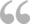


Leave a comment